A mental health disorder is defined as any condition that affects a person’s thoughts, behaviors or moods. Learn more about common mental illnesses as well as causes, diagnosis and treatment.
Mental health disorders are common conditions, affecting an estimated 54 million Americans each year. Mental health conditions can cause frequent stress and can be both emotionally and physically trying. If you or a loved one is struggling with mental health concerns, know that you are not alone and help is available.
What Is Mental Illness?
A mental health disorder is defined as any condition that affects a person’s thoughts, behaviors or moods. While some mental health disorders last for a limited period, others are chronic and lifelong. When these issues cause high levels of stress or affect their daily functioning or relationships, treatment may be necessary to help a person manage their symptoms.

Common Mental Health Disorders
A few of the most commonly diagnosed mental health disorders include the following:
Treatment Can Be Life Changing. Reach out today.
Whether you are struggling with addiction, mental health or both, our expert team is here to guide you every step of the way. Don’t wait— reach out today to take the first step toward taking control of your life.

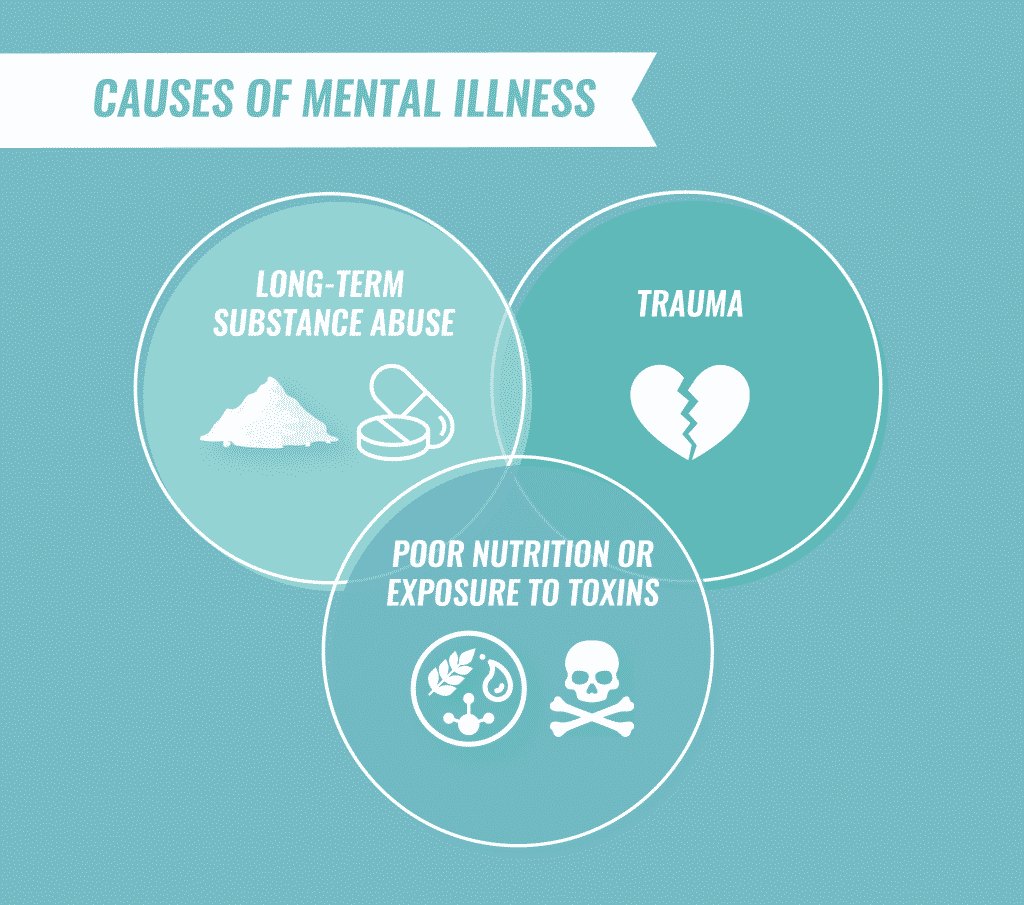
Causes of Mental Illness
Although the exact cause of most mental illnesses is unknown, most develop as the result of a combination of biological, psychological and environmental factors.
Some mental illnesses have been linked to abnormal functioning of the brain due to chemical imbalances, injuries or developmental abnormalities. Mental illnesses sometimes run in families, suggesting that genetics also plays a role. Other links to mental health disorders include:
- Long-term substance abuse
- Poor nutrition and exposure to toxins
- Undergoing severe psychological trauma as a child, including emotional, physical or sexual abuse
- Death or divorce
- Dysfunctional family life
- Feelings of inadequacy, low self-esteem, anxiety, anger or loneliness
- Social or cultural expectations
- Substance abuse
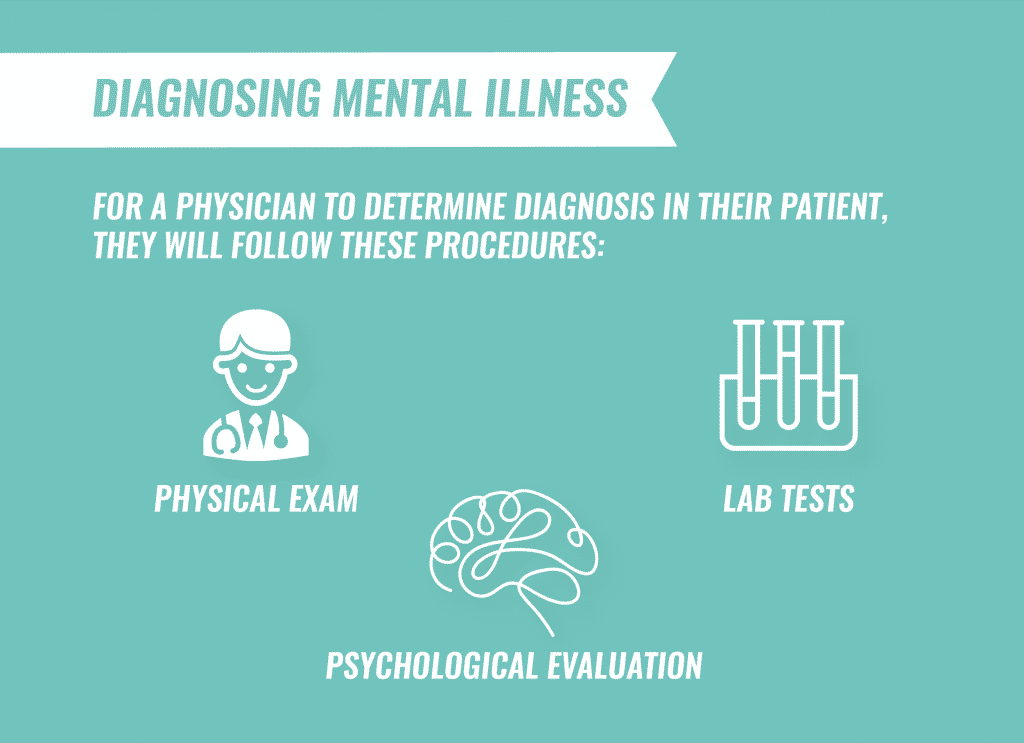
Diagnosing Mental Illness
Physicians will typically check for related complications while diagnosing a mental health disorder and perform:
- Physical exams to rule out any physical problems that could be causing the symptoms
- Lab tests to evaluate body processes or screen for alcohol and drugs
- Psychological evaluation to assess mental illness symptoms, thoughts, feelings and behavior patterns
Statistics on Mental Illness
Mental health disorders are one of the most common causes of disability in the United States and bear the largest disease burden of any category of health conditions. An estimated54 millionAmericans live with a serious mental illness or mental health issues in any given year.
Mental illness also includes alcohol and substance use disorders. In 2013, approximately17.3 million Americansover the age of 12 lived with an alcohol use disorder in the past year. Roughly 6.9 million Americans 12 and older abused illicit drugs and were dependent on them in the year before being surveyed.

Mental Health and Substance Abuse
Co-occurring disorders, ormental health and substance use disorderspresenting simultaneously, are exceedingly common. People living with a drug or alcohol use disorder are about twice as likely to already exhibit symptoms of a mental health disorder. Similarly, those who are living with a mental health disorder are twice as likely to develop a substance abuse problem as well.
Mental Illness Stigma
Some individuals still view mental illnesses as threatening. These views can lead to various forms of exclusion and discrimination for people with mental health problems.
Some of the additional harmfuleffects of stigmacan include:
- Reluctance to seek help or treatment
- Lack of understanding by family, friends, co-workers or others
- Fewer opportunities for work, school or social activities
- Trouble securing housing
- Bullying, physical violence or harassment
- Health insurance that doesn’t adequately cover mental illness treatment
Mental Health Treatment
Treatments may vary depending on the type of mental health disorder a person has. However, mental health care almost always involves some form of psychiatric counseling. Medications may also be prescribed.
If you or a loved one is living with co-occurring addiction and mental health disorders that are affecting your life, The Recovery Village can help. Individuals with co-occurring mental health and substance use disorders can receive comprehensive treatment from one of the facilities located across the country. To learn more, call The Recovery Village today tospeak with a representative.
Mental Health Conditions
- Acute Stress Disorder
- ADHD/ADD
- Adjustment Disorders
- Agoraphobia
- Anorexia
- Antisocial Personality Disorder
- Anxiety Disorders
- Austim Spectrum Disorder
- Avoidant Personality Disorder
- Avoidant Restrictive Food Intake Disorder
- Binge Eating
- Bipolar Disorder
- Body Dysmorphic Disorder
- Body-Focused Repetitive Behaviors
- Borderline Personality Disorder
- Bulimia
- Circadian Rhythm Sleep-Wake Disorder
- Claustrophobia
- Codependency
- Conversion Disorder
- Cyclothymic Disorder
- Dementia
- Dependent Personality Disorder
- Depersonalization-Derealization Disorder
- Depression
- Diabulimia
- Disinhibited Social Engagement Disorder
- Disruptive Behavior Disorder
- Disruptive Mood Dysregulation Disorder
- Dissociative Amnesia
- Dissociative Disorders
- Dissociative Fugue
- Dissociative Identity Disorder
- Dysthymia
- Eating Disorders
- Excoriation
- Factitious Disorder
- Gender Dysphoria
- Generalized Anxiety Disorder
- Grief
- Histrionic Personality Disorder
- Hoarding
- Hypersomnia
- Hypomania
- Imposter Syndrome
- Impulse Control Disorder
- Insomnia
- Kleptomania
- Major Depressive Disorder
- Mania
- Mood Disorders
- Narcissistic Personality Disorder
- Narcolepsy
- Nightmare Disorder
- OCD
- OCPD
- Orthorexia
- OSFED
- Panic Disorder
- Paranoid Personality Disorder
- Personality Disorders
- Phobias
- Pica
- Postpartum Depression
- Premenstrual Dysphoric Disorder
- Process Addiction
- Psychosis & Psychotic Disorders
- PTSD
- Pyromania
- Reactive Attachment Disorder
- REM Sleep Behavior Disorder
- Rumination
- Rumination Disorder
- Schizoaffective Disorder
- Schizoid Personality Disorder
- Schizophrenia
- Schizotypal Personality Disorder
- Seasonal Affective Disorder
- Self-Harm
- Separation Anxiety Disorder
- Sleep Disorders
- Social Anxiety Disorder
- Somatic Symptom Disorder
- Somatoform Disorders
- Stress
- Tourette’s
- Trichotillomania

Time's up







